

Introduction to GSC No.4
Revised Edition
Received the Minister of Economy, Trade and Industry Award of the 14th GSC Awards (2013)
Development and Commercialization of High Performance Transparent Plastics Derived From Plant-Based Raw Material
Mitsubishi Chemical Corporation
This page contains part of the PDF version.
Please see the PDF version for details.
Mitsubishi Chemical Corporation succeeded in the development and commercialization of transparent engineering plastics whose main raw material is isosorbide derived from renewable resources. Not only was the environmental impact reduced by using a unique process utilizing renewable resources, but the performance of the product, such as excellent impact resistance and weathering resistance, was radically improved as well.

DURABIOTM (left) and a car body fabricated using DURABIOTM
(right: image provided by Mazda Corporation)
Outline of award-winning company
Mitsubishi Chemical Corporation has developed petrochemicals, etc. as a comprehensive chemicals manufacturer. In 2017, the company merged with Mitsubishi Plastics and Mitsubishi Rayon to form Mitsubishi Chemical Corporation (Head office: Chiyoda-ku, Tokyo).
The Path to Technology Development
What were the intentions that started development toward realizing the sustainable progress of society?
Plastics are widely used to manufacture daily necessities, electrical parts, and packaging materials, and have become indispensable to modern society. At present, almost all plastics are derived from petroleum-based raw materials, which are associated with several environmental issues (such as the exhaustion of resources and a high environmental impact during disposal). Consequently, it is crucial to promote the practical application of plastics with a low environmental impact, such as those synthesized using raw materials from renewable organic resources and biodegradable plastics.
Mitsubishi Chemical Corporation aims to make a conversion to "zero fossil resources" by developing plastics from bio-based raw materials. On the other hand, the company offers an impressive lineup of products and engineering plastics with excellent mechanical strength and heat resistance, and has developed a wide range of products that meet the needs of the market and consumers. Among these products, polycarbonate, which is used to fabricate DVDs and CDs, exhibits a high impact resistance (250 times greater than that of glass) along with excellent transparency, heat resistance, and dimensional stability. However, polycarbonate has disadvantages such as optical anisotropy and a surface prone to scratching. To eliminate these disadvantages and "create a novel material for replacing glass," the polymer structure of polycarbonate was re-examined.
Polycarbonate refers to a polymer having a carbonate group, and the polycarbonate which is commonly used today is an aromatic polycarbonate made from polymerization of bisphenol A.

Structures of polycarbonates and DURABIOTM
Plastics containing aromatic rings are characterized by large birefringence. Therefore, aliphatic compounds which do not contain aromatic rings can be used to improve the optical characteristics of a material.
Aliphatic polycarbonates synthesized from aliphatic monomers exhibit a low melting point and softening point, and cannot be used as practical molding materials. The excellent properties of aromatic polycarbonates are attributed to the bisphenol A monomer. Is it possible to create a new material with excellent physical properties without using bisphenol A? To resolve this issue, the development team in Mitsubishi Chemical Corporation began investigating suitable monomers (alternatives to bisphenol A) for the process.
Towards Resolution of Issues
What kind of technological challenges did the developers face, and how did they come up with solutions?
Searching for an excellent monomer to replace bisphenol A
Polycarbonate synthesis can occur via an "interfacial method" that involves interfacial polycondensation and a "melting method" that involves transesterification (refer to
Introduction to GSC No.2). The interfacial method, which utilizes bisphenol A and phosgene, is commonly used. However, general aliphatic compounds cannot be polymerized by the interfacial method because of their properties; thus, the melting method is used for this process. In the melting method, the transesterification of alcohol (in this case, aliphatic diol) with diphenyl carbonate (an ester) is used to synthesize polycarbonates. This method not utilizing a large amount of solvent and exhibits a lower environmental impact than the interfacial method.
However, the reactivity and properties of polymers synthesized from aliphatic diols vary with the structure of the monomer used. Straight-chain aliphatic polycarbonate using straight-chain aliphatic diol has a structure in which the molecules move easily, causing the heat resistance to be insufficient. On the other hand, if annular aliphatic diol is used as the raw material, the reactivity drastically decreases and the polymerization reaction will not proceed.
A tradeoff relationship exists between polymerization and heat resistance. After investigating several aliphatic diols for high heat resistance and good reactivity, "isosorbide" was identified as optimal for polycarbonate synthesis.

Production of isosorbide from glucose
The reduction of glucose (from starch) generates sorbitol, which is used to synthesize isosorbide.
Isosorbide, derived f rom starches and sugars from plants such as corn and wheat, is commonly used a raw material for pharmaceuticals such as diuretic drugs. Isosorbide exhibits a secondary heterocyclic diol structure that contains carbon atoms and an oxygen atom. As isosorbide is not a straight-chain aliphatic diol, polymers derived from isosorbide exhibit acceptable heat resistance and stiffness. Moreover, the oxygen atom in the ring structure imparts high reactivity to polycarbonates derived from isosorbide.
Thus, the development of high-performance engineering plastics using renewable resources began by integrating two research projects on "making plastics from bio-based raw materials" and "making strong, novel engineering plastics with high transparency like glass that are resistant to breaking."
Brittleness, brown color - issues raised one after another
Although it was finally found that isosorbide is a monomer which has both heat resistance and reactivity, there was a large number of issues before the completion of the resin. First, when a polycarbonate with a structure having only isosorbide as the diol was synthesized, the resulting polymer had low flexibility and was brittle. The result was far inferior to the physical properties of aromatic polycarbonates. The molecular design had to be redone again.
Subsequently, the copolymerization of isosorbide with other aliphatic diols was proposed. The physical properties of the polymer were calculated by a computer, and investigations were made to improve brittleness while maintaining heat resistance. As a result, it was found that the glass transition temperature could be adjusted by changing the type of copolymerization components and the ratio of copolymerization with isosorbide.
During product development, experiments are first developed in the laboratory and then repeated in progressively larger facilities. Even if an experiment is successful on a small scale, unexpected side reactions may occur when the scale of an experiment is increased. In this case, it became clear that the synthesized polymer developed a brown color and produced impurities. Moreover, the melting method requires reactions that last for a long period of time at high temperature, but the yield must be increased by efficient reactions for commercial production.
Therefore, a simulation of the polymerization reaction was utilized, and the catalyst was improved by using alkaline earth metals such as calcium and magnesium. After meticulously re-examining the polymerization process, several improvements (such as stabilizer optimization) were carried out. In the production process, the raw material isosorbide is melted and mixed with the copolymerization component aliphatic diol, and diphenyl carbonate. Phenol, which is produced as a by-product, is subjected to pressure reduction and removed from the system in the presence of a catalyst, enabling the polymerization reaction to proceed. It was found that if the polymerization is carried out at a high temperature in high vacuum from the start, the raw material became distilled out of the system before the reaction took place, and the polymerization ceased to proceed midway.
Thus, in the early stages of polymerization, the reaction proceeded at a comparatively low temperature in low vacuum. Toward the end of the reaction, the phenol concentration in the system was reduced at a comparatively high temperature in high vacuum, and macromolecule quantification occurred on shifting the equilibrium toward the polymerization side. In an industrial aspect, production is carried out by using several reactors with different reaction conditions in the early and final stages of the polymerization.
In the thus completed process, the reaction time was shortened and the reaction could be proceeded at a low temperature. The coloring of the resin is caused by the low thermal stability of isosorbide, and the resin becomes further prone to coloring at a high temperature. When it became possible to make the polymerization proceed in a short time even at a low temperature, a colorless resin could be obtained, and reduction of energy also became possible.
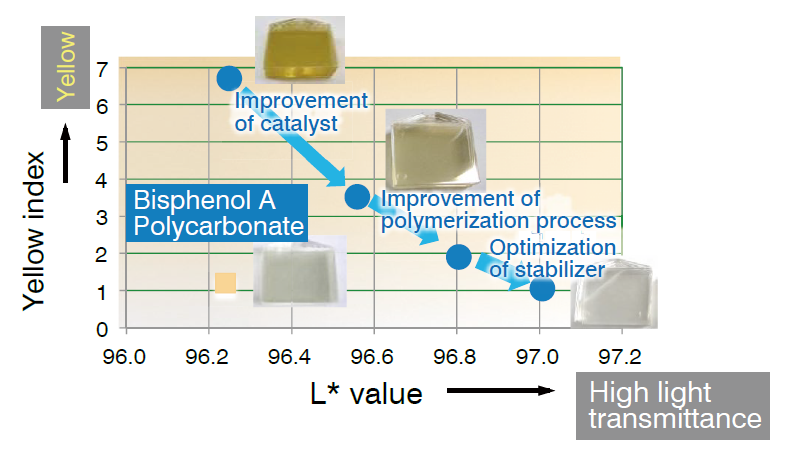
Process improvements
High-quality resins with high transparency were synthesized by tuning the polymerization conditions.
The L* value indicates the brightness of the color (lightness); a larger value indicates a higher color tone.
Using a plant-based raw material will amount to nothing i f the process causes a large environmental impact. By reusing purified by- product phenol as a raw material for diphenyl carbonate, it is possible to produce it in a completely closed cycle without emitting emissions. Minute amounts of impurities are used as heat sources for the boiler.

A completely closed cycle
After melting and mixing diphenyl carbonate, isosorbide, and an aliphatic diol, phenol (generated as a by-product) was removed from the system by pressure reduction; this facilitated the polymerization reaction.
Contribution to Society
What is the contribution of this novel technology to society?
The developed resin was named DURABIOTM and put on the market. DURABIOTM uses isosorbide as the main starting material and contains carbons derived from CO2 in the atmosphere within its framework. Therefore, the CO2 produced by burning the resin does not increase the amount of CO2 in the atmosphere, and the exhaustible resources emitted during the disposal process generate low CO2 emissions.

The low environmental impact of DURABIOTM
Left: Environmental impact index calculated according to the Green Value Chain Platform of the Ministry of the Environment (serves as an indicator of LCA: life cycle assessment)
Right: Comparison of environmental impactindexes with existing polycarbonates Green Value Chain Platform of the Ministry of the Environment
https://www.env.go.jp/earth/ondanka/supply_chain/gvc/en/
Furthermore, the development of a hyperactive catalyst and improvement in the production process, which enabled polymerization at a low temperature and in a short time, led to the reduction in primary energy usage, i.e., fossil resources usage. Furthermore, not using solvents such as methylene chloride and realizing a completely closed cycle have resulted in drastic reduction in environmental impact.
Unlike conventional polycarbonates , DURABIOTM exhibits high transparency , weathering resistance, and resistance to scratching. And despite being a plant- based polymer, it is not biodegradable but is rather highly durable. It has excellent optical properties and is comparable to acrylic resins which are transparent, highly durable and used as a substitute for glass. It exhibits ductility which is not a property of acrylic resins, and thus its application is expected to expand to optical films, etc., in addition to applications utilizing the initially-sought high transparency. Furthermore, owing to negligible yellowing due to UV light, it can be used for outdoor-type sheets and surface films that require resistance to weathering.

Characteristics of DURABIOTM
A high-performance resin was derived from a plant-based raw material with novel functions (such as excellent optical characteristics and germ repellency). This new resin exhibits the heat resistance and impact resistance of polycarbonates and the high transparency and UV resistance of acrylic resins.
Although DURABIOTM (with the advantageous properties of both polycarbonates and acrylic resins) showed several potential applications, its optical-property-based applications expanded slowly, possibly owing to its high cost. However, an unexpected application in automobile parts was discovered while advancing development to answer to customers' requests. Since DURABIOTM is a highly transparent resin, it exhibits good color development, and was thus appraised for being able to express "smoothness like a mirror surface and deep hues" that surpassed the conventional coated parts, simply by adding pigments. Moreover, its hard surface and resistance to abrasion make the painting process unnecessary, reducing the volatile organic compounds (VOCs) generated from paints, and it is a thermoplastic resin that can be melted and reused, even if the molding fails.
DURABIOTM also showed a germ-repelling ("Germrepelling") property; it repelled microorganisms that adhere to the material surface and enabled their facile removal by washing with water. Although the clarification of the detailed mechanism is still in the works, its application is expected to expand to medical equipment, etc.
The development of high performance resins using renewable resources probably would not have been realized without the design and production technologies for resin established by the company over the years, in addition to the efforts of the persons in charge. Moreover, the marketing power of the company enabled an expansion of the applications of DURABIOTM. However, issues still remain. The raw materials for isosorbide are edible materials such as corn, but must be switched to inedible raw materials in the future. Furthermore, continuing efforts are made for a production process with further reduced environmental impact.
This page contains part of the PDF version.
Please see the PDF version for details.






